Going Global | NCC Medical Successfully Passes TUV CE/MDR On-site Audit
NCC Medical Passes International Authoritative Audit, Accelerating Global Market Layout
NCC Medical, located in Shanghai Pudong International Medical Industry Park, on September 24, 2025 , an audit expert team from TÜV SÜD officially commenced an on-site factory audit for CE MDR compliance at Shanghai NCC Medical Co., Ltd. This rigorous audit, conducted by the internationally authoritative third-party organization TUV Rheinland, marks a critical step for NCC Medical towards obtaining CE MDR certification and is a milestone for the company's products to enter the EU, Singapore, and other global high-end medical markets.

To fully meet the stringent requirements of the CE MDR regulations for high-risk medical devices, NCC Medical has systematically restructured its entire medical device lifecycle management system from research and development to production and delivery over the past three years, covering core aspects such as technical documentation, clinical evaluation, risk management, and production control. This ensures product safety, effectiveness, and traceability, fully complying with the MDR's general safety and performance requirements.

Focus on Process: Compliance in All Processes, Strengthening the Foundation for CE MDR Certification
In the R&D phase, the company strictly adheres to the EN 60601 standards and CE MDR requirements, establishing a risk-based design control process. From product concept initiation to design changes, each step undergoes review, verification, and validation, forming a complete and traceable technical documentation package that meets TUV Rheinland high standards for completeness and compliance.
In terms of clinical validation, in accordance with relevant CE MDR regulations, the company conducted multi-center, prospective clinical evaluation studies, systematically collecting clinical data to fully demonstrate the product's clinical benefits and controllable risks, providing solid evidence support for CE certification.

Regarding quality control and the production system, the company has fully implemented a quality management system compliant with ISO 13485:2016 and EU GMP requirements. NCC medical builds a Class 10,000 Clean Production Workshop, implements multi-level inspection procedures for incoming materials, semi-finished products, and finished products, and strictly enforces process validation and continuous monitoring throughout production. This ensures each device is stable, reliable, conforming to specifications, and satisfies TUV's audit standards for production consistency and process control.

Specialization: "Equipment + Consumables" Integration, Setting a Benchmark for Chinese Intelligent Manufacturing
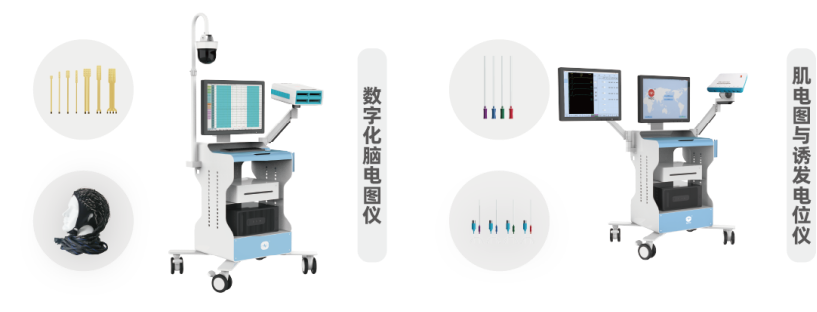
It is particularly noteworthy that NCC Medical is one of the few companies in the global neurophysiology industry capable of producing both professional medical devices and compatible consumables. The company not only masters the core technologies of EEG, EMG, evoked potential systems, and multi-functional neural monitors but also, through its subsidiary Shanghai Handy Medical Instrument Co., Ltd., independently designs and produces a full range of intraoperative neuromonitoring consumables, including nerve stimulation electrodes, recording electrodes, cortical electrodes, urological electrodes, suction probes, EEG electrodes, connection wires and more.
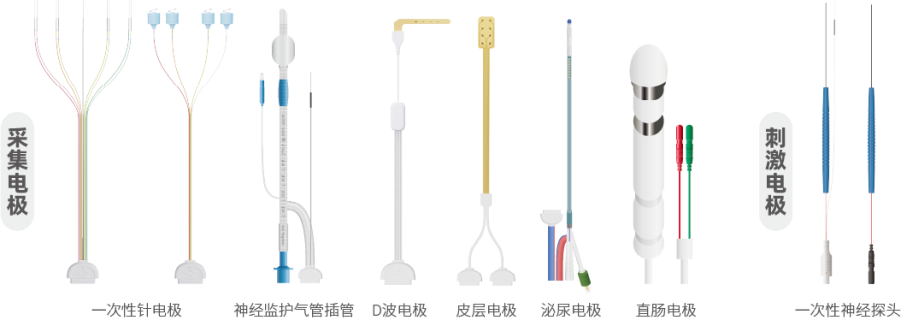
The quality of all key processes, from material selection, mold development, injection molding to circuit packaging and final assembly, are independently controlled. This "equipment + consumables" production model ensures high compatibility of signal interfaces, electrical parameters, and anti-interference performance. NCC medical conducts integrated machine testing and compatibility verification in the early stages of R&D, and performs system-level functional inspections before shipment, fundamentally preventing signal distortion or monitoring interruptions caused by incompatible third-party consumables, significantly enhancing the stability and safety of the intraoperative system – an advantage that also earned high attention and recognition from the TUV audit experts.
Technical Breakthrough: Overcoming Surgical Environment Interference Challenges, Enhancing CE MDR Product Reliability
Addressing the interference challenges posed by complex electromagnetic environments in operating rooms, the company has established a high-standard EMC anti-interference laboratory. This lab simulates scenarios with multiple strong interference sources like high-frequency electrosurgical units and patient monitors coexisting, conducting full-condition immunity tests on products. Relying on this platform, the R&D team continuously optimizes shielding structures, filter circuits, and differential amplification algorithms to ensure stable acquisition of microvolt-level neural signals even under extreme interference conditions.

Verified by third-party testing, NCC Medical products’CMRR and transient interference suppression capabilities achieve at internationally advanced levels, significantly improving the accuracy and reliability of intraoperative monitoring. These key performance indicators are precisely the technical parameters TUV focuses on during CE MDR audits and are core basis for product certification under the EMC Directive 2014/30/EU.

Leading Industry Upgrade: Pioneering Class III Registration in China, Responding to New National Regulations
In 2024, NCC Medical took the lead among domestic peers by completing the registration category upgrade for its intraoperative neural monitoring system, successfully registering its core product Intraoperative Neurophysiological Monitoring Equipment as a Class III medical device. This made it the first domestic company to achieve Class III registration for such product.
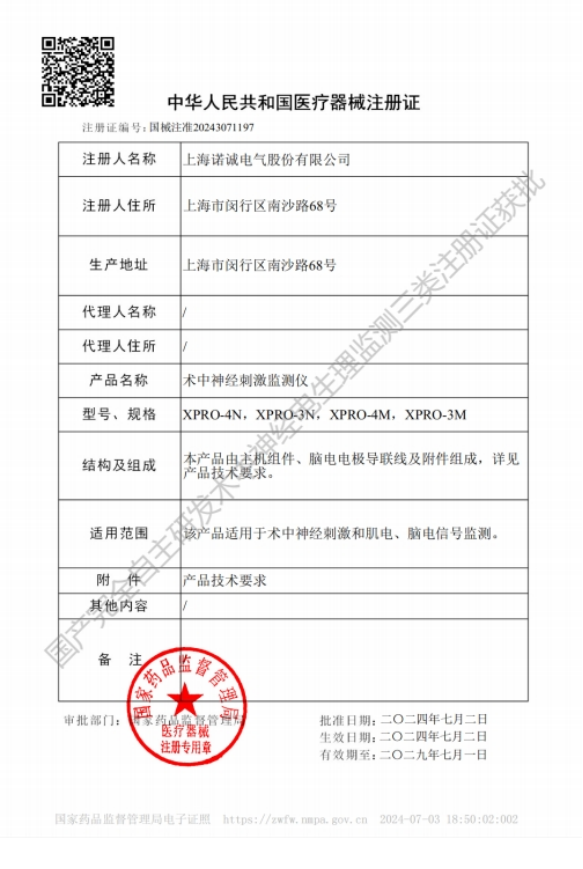
This move aligns with the requirements of the NMPA's announcement on adjusting the classification management of some medical devices, which stipulates that "devices used for real-time intraoperative monitoring of neurological function, directly influencing surgical decisions" be managed under the highest risk classification. This not only demonstrates the company's proactive alignment with the "risk-based classification and supervision" concept emphasized by CE MDR but also lays a solid foundation for building clinical evidence in subsequent international registrations.
Quality System: Guided by International Standards , Building a Foundation for Sustainable Development
Since its establishment, NCC Medical has always regarded "Quality First" as the lifeline of its development. Our company first passed ISO 13485 quality management system certification in 2010 and has continuously maintained the effective operation and dynamic improvement of the system. In recent years, the company has invested significant resources in upgrading its Quality management system(QMS), achieving electronic traceability and risk early warning across the entire process from design and development, procurement, production, to after-sales service.
"This CE MDR factory audit by TÜV is not only a comprehensive test of our existing system but also an important opportunity to drive the company towards globalization, standardization, and lean management," said the Quality System Principle at NCC Medical. "We look forward to using this audit as a starting point to accelerate the completion of CE MDR certification, bringing China's independently developed high-end electrophysiology equipment to a broader international market, and providing safer, smarter neural monitoring solutions for global patients and clinicians."
NCC Medical's International Compliance Process Advances Steadily
Previously, Shanghai NCC Electronic Corp.,Ltd. and its consumables subsidiary Shanghai Handy Medical Instrument Co.,Ltd. had respectively passed ISO 13485:2016 quality management system certification issued by DEKRA(Certificate No.6162750), laying a solid foundation for global market access.
In June 2025, NCC medical's core product Cynapse IONM (for Neurosurgery/Orthopedics) successfully passed an on-site audit by Brazil's INMETRO at the NCC Medical Park in Shanghai Pudong and obtained certification, marking the product's formal entry into the high-end medical market of South America. Subsequently, in September, the product successfully obtained the registration certificate(Certificate No. 0989678253) from the Brazilian National Surveillance Agency (ANVISA), achieving comprehensive access from system to product.

Next, another product Smart IONM (for ENT surgeries) will undergo a factory audit from INMETRO in Brazil. This product had already obtained registration from Colombia's INVIMA(Certificate No.2024DM-0029185) in 2024 and has achieved commercial deployment locally.

Within the year, the company will also undergo an on-site factory audit by Russian regulatory authorities, steadily advancing its compliance strategy in the CIS market. It is worth mentioning that NCC Medical has assisted its Indian partner in successfully obtaining the Indian CDSCO registration certificate (Certificate No.: MFG/MD/2024/000065) in June 2025, achieving a breakthrough in the " Intelligently Manufactured in China, Delivered Globally" cooperation model.

Meanwhile, the localization registration process in various countries worldwide is accelerating comprehensively. Product registration and local certification in South Korea, Indonesia, Malaysia, Mexico, Peru, Ecuador, South Africa, and other countries have officially commenced and entered the queuing process. This signifies that NCC Medical is systematically building a global compliance layout covering Asia-Pacific, Latin America, Europe, and other emerging markets, steadily advancing the internationalization of high-end "Intelligently Manufactured in China" medical devices.
About Shanghai NCC Medical Co., Ltd.
Founded in 1997, Shanghai NCC Medical Co., Ltd. has long focused on the R&D, production, and service of high-end medical equipment for intraoperative neural monitoring, EEG monitoring, electromyography, etc. Our company has over 300 employees, and our products have covered more than 5,000 medical institutions across China, widely used in tertiary hospitals and regional medical centers, while continuously expanding into overseas markets including Southeast Asia, Europe, the Americas, and Africa.

Recognized as a National-Level Specialized, Refined, Characteristic, and innovative “Little Giant” Enterprise by the Ministry of Industry and Information Technology of China, NCC Medical adheres to independent innovation and lean manufacturing, and has built a fully self-controlled chain from core technology R&D to key component production. Its consumables subsidiary, Shanghai Handy Medical Instruments Co., Ltd., has also been recognized as a Shanghai Municipal Specialized, Refined, Characteristic, and innovative “Little Giant” Enterprise, further strengthening the company's leading advantage in equipment and consumables synergistic innovation.

Our company places high importance on technological innovation and clinical value. Its independently developed IONM systems and 256-channel Digital EEG System with Event-Related Potentials for auxiliary cognitive diagnosis have both been selected into the Shanghai Innovative Medical Device Catalog, signifying authoritative recognition of the products' technological advancement, clinical necessity, and quality safety level.
Leveraging its professional EMC anti-interference laboratory and the unique dual production bases for both "equipment and consumables," NCC Medical continuously overcomes electromagnetic interference challenges in surgical environments, enhancing the stability and reliability of intraoperative monitoring. In 2024, the company took the lead in completing the Class III registration upgrade for its intraoperative neural monitoring system, actively responding to the national policy direction of strengthening supervision of high-risk medical equipment and leading the industry in compliance and high-quality development.


In the future, NCC Medical will continue to uphold the development philosophy of "Innovation-Driven, Quality-Based," committed to promoting Chinese high-end medical devices to the world, providing safer and smarter comprehensive neurophysiological solutions for global patients and clinicians. Taking the successful TUV CE MDR audit as a new starting point, the company will accelerate its international strategic planning.

 中文
中文 Arabic
Arabic Spanish
Spanish Hindi
Hindi French
French Indonesian
Indonesian Portuguese
Portuguese Persian
Persian Russian
Russian Korean
Korean German
German Vietnamese
Vietnamese Turkish
Turkish